EN 1040:2005
Quantitative suspension test for the evaluation of basic bactericidal activity of chemical disinfectants and antiseptics.
EN 1040 is a phase 1 suspension test for the evaluation of basic bactericidal activity in chemical disinfectants. The result of a basic suspension test cannot be used to substantiate product claims and it is not valid for product registration. The test is applicable to active substances (antibacterial biocides) and to formulations under development that are planned to be used in food, industrial, domestic and institutional, medical and veterinary areas.
Test Conditions
The standard refers to the parameters to be observed when testing products for basic bactericidal activity. This includes the test microorganism, test temperature and contact time.
- Test microorganism refers to the mandatory list of microbes that must be used in the test to determine the antimicrobial activity of the product. The mandatory microorganisms are assumed to represent all microbes in its group.
- Test temperature refers to the temperature in which the test must be conducted. The general assumption is that disinfectants are less effective in low temperatures compared to higher temperatures.
- Contact time refers to the minimum duration a product must remain in contact with the microbes for the product to be effective.
One of the major differences between phase 1 basic suspension tests and phase 2 step 2 suspension tests is that phase 1 basic suspension tests do not include interfering substances in the test procedure.
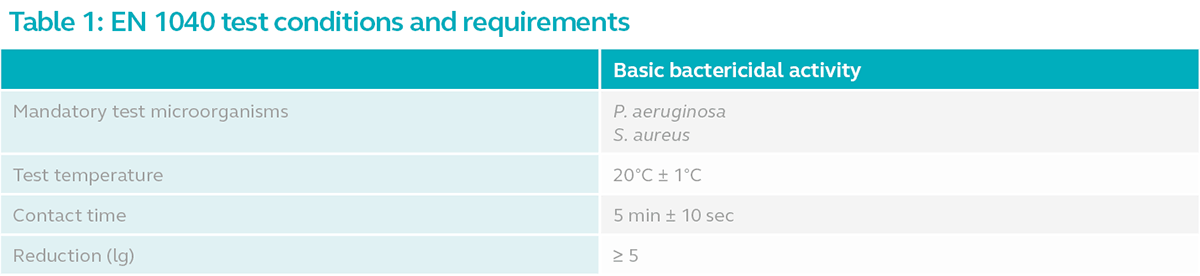
Refer to the table below for the minimum test conditions for EN 1040.
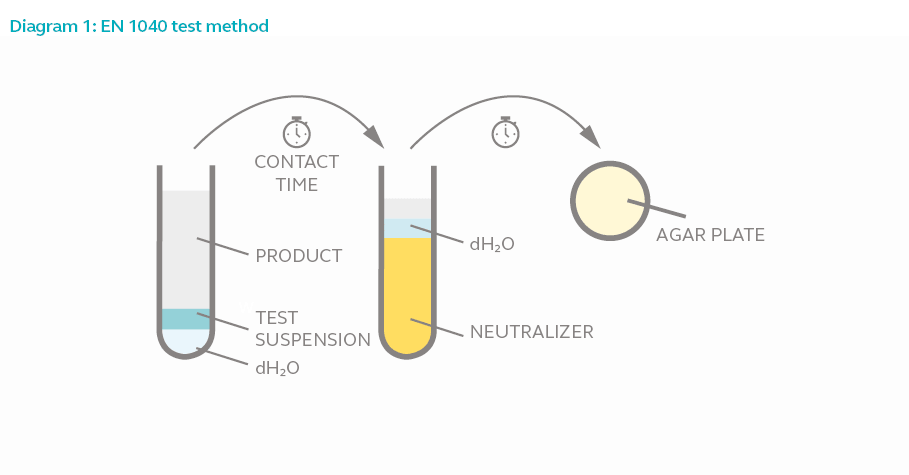
Test Method
In a phase 1 suspension test, 8 parts of the test product is added to 1-part test microorganism and 1-part water. The mixture is allowed to interact for the duration of the contact time. One part of the mixture is added to 8 parts of neutralizer and 1-part water for 5 minutes to halt bactericidal activity. The final mixture is then acquired and incubated for 2 days to allow surviving bacteria (if any) to proliferate. The bacterial colony is counted and compared against the original culture size.
Log Reduction
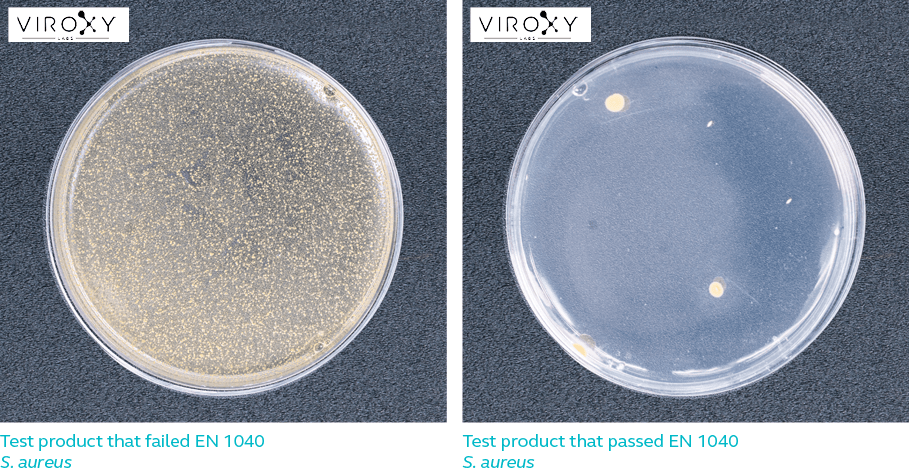
Log reduction refers to the extent to which a product is capable of reducing the number of microbes. For example, 4-log reduction means the number of microorganisms on a surface has been reduced by 10 000 times. A product that is 99.9% effective against a certain microbe is said to have achieved 3-log reduction against that microbe.
For a product to pass EN 1040, it must be able to achieve 5-log reduction against the respective test microorganisms listed in Table 1. In other words, the product must be able to kill 99.999% bacteria while meeting all the other requirements of the European standard.