EN 14476:2013+A2:2019
Quantitative suspension test for the evaluation of virucidal activity of disinfectants intended for use in the medical area.
EN 14476 is a phase 2 step 1 suspension test to evaluate the virucidal activity of chemical disinfectants intended for use in the medical area.
Test Conditions
Refer to the table below for the minimum requirements for EN 14476 test:
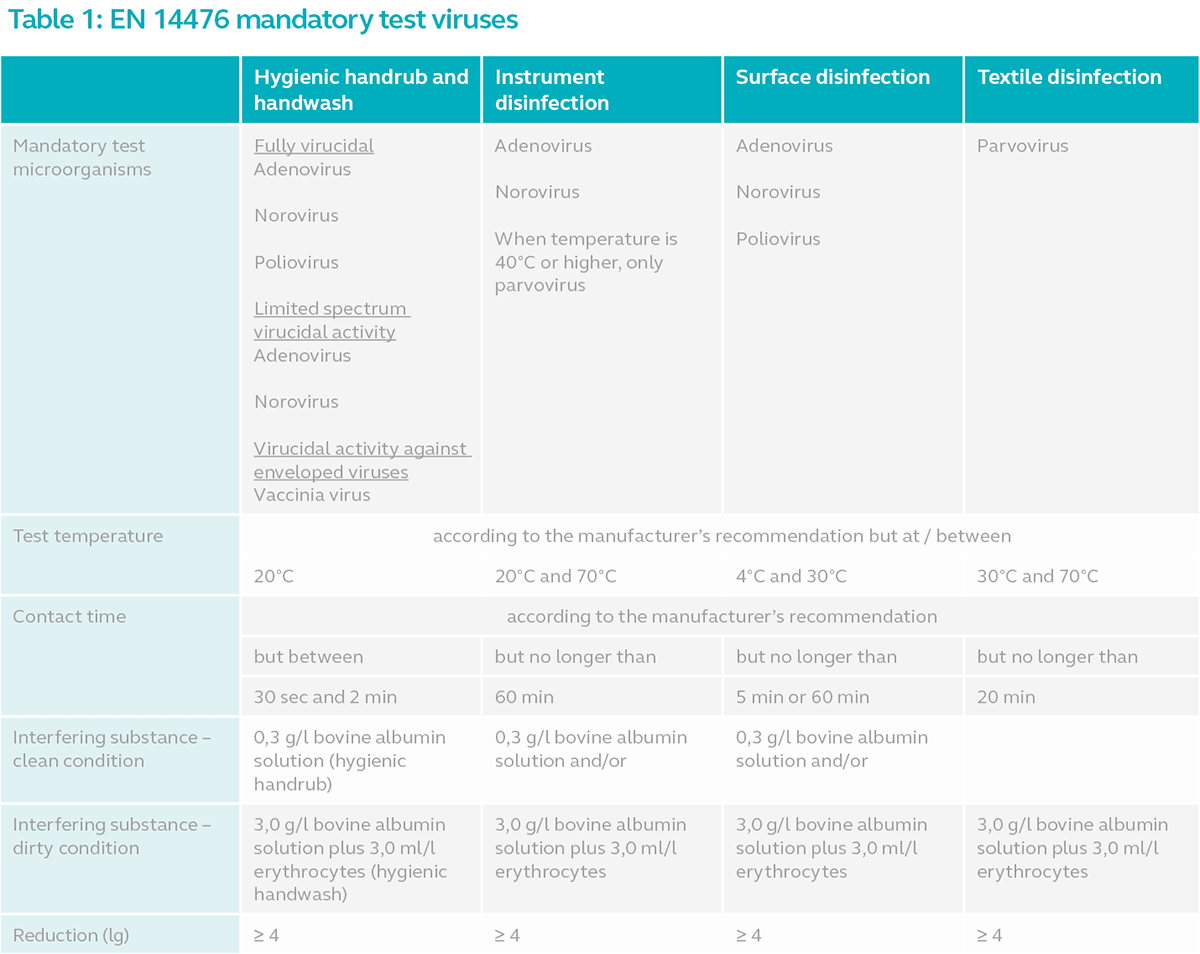
All the viruses listed are non-enveloped viruses (except vaccinia virus) and as such are more resistant to chemical disinfectants compared to enveloped viruses. Poliovirus is the most resistant among non-enveloped viruses and manufacturers often struggle to attain efficacy against this microorganism. If EN 14476 is performed against all three non-enveloped viruses above for a hand product and it passes the test for adenovirus or norovirus but fails against poliovirus, the product is deemed as limited spectrum virucidal or limited virucidal. For a hand product to be fully virucidal or acknowledged as capable of inactivating all enveloped and non-enveloped viruses, it must be effective against adenovirus, norovirus and poliovirus. Instrument and surface disinfectants intended for the medical area however, must pass the test against all three non-enveloped viruses.
Test Method
In preparing for the suspension test, the test virus is added to an interfering substance in a suspension. The choice of interfering substance used in the test depends on the product claim. The test product or disinfectant is then added to the virus suspension for the duration of the exposure time at the temperature specified by the manufacturer.
At the end of the exposure time, samples are retrieved and the activity of the test product is neutralised by dilution in ice-cold test medium. Serial dilutions are performed, and the dilutions are examined for viral infectivity.
Unlike bacteria or fungus, most viruses are too small (ranging from 25nm to 400nm) for observation under a light microscope. The presence of viruses in a suspension before and after product exposure is therefore determined by inoculating live host cells with suspension samples. These cells are then observed after 7 days (depending on the cell type) for structural changes. If the test product had not been successful in inactivating test viruses before neutralization, they invade and damage the live cells to display cytopathic effect (CPE). These are the effects virologists look for when observing the cells under a light microscope.
Control Tests
In addition to the efficacy test, 5 control tests are run concurrently to eliminate other possible explanations for the test results. The 5 control tests are:
1. Virus control
Determines the infectivity of the test virus suspension. To pass the test, the concentration of virus in the control test must be sufficiently high to enable a 4-log reduction or reduction of viruses by 10 000-fold.
2. Cytotoxicity control
Reveals the possible alteration in cell structure caused by the test product or disinfectant. To pass the test, live cells must not display toxic reaction or damage to a level where achieving 4-log reduction is not possible.
3. Suppression control
Verifies the efficiency of the neutralising method in suppressing the virucidal activity of the test product after the required exposure time.
4. Interference control
Verifies the susceptibility of infection in cells is not influenced negatively by the test product (must have passed cytotoxicity test).
5. Reference control (using formaldehyde)
Ensures the test virus can be inactivated and non-resistant to antimicrobial agents, enabling it to achieve 4-log reduction.
Determination of Results
CPEs if present, can be viewed through a light microscope and the reduction in virus infectivity is calculated through the difference in virus concentration before and after treatment with the test product. A 4-log reduction or reduction of viruses by 10 000-fold demonstrates the ability of the test product in inactivating viruses to a level acceptable to the European standards. Log reductions are calculated by determining 50% Tissue Culture Infective Dose (TCID50) or the viral dose required to display CPE in 50% of the cell culture.