TGA Disinfectant Test
TGA Disinfectant Test should be your first focus when expanding your disinfectant business to the Land Down Under.
TGA Disinfectant Test refers to the suspension test used in Australia to determine the bactericidal activity of disinfectants. The European equivalent of this test include:
- EN 13727:2012+A2:2015 – Quantitative suspension test for the evaluation of bactericidal activity in the medical area.
- EN 1656:2019 – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants used in the veterinary.
- EN 1276:2019 – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas.
The manufacturing and marketing of disinfectants in Australia is guided by Therapeutic Goods (Standard for Disinfectants and Sanitary Products) (TGO 104) 2019.
Product Classification
In Australia, disinfectants are divided into two classes:
- Hospital grade
- Household or commercial grade
The bactericidal activity in each class of product is tested using 1 of 3 test options. The requirements for each option are summarised below:
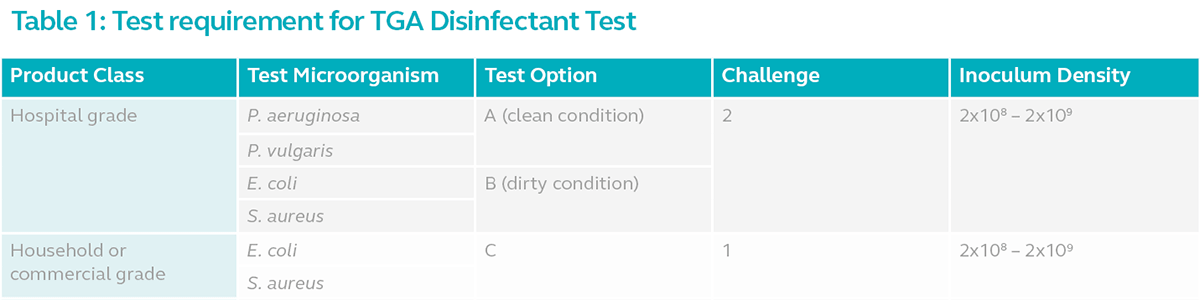
Disinfectants intended for use in healthcare facilities must be tested according to option A or B with 2 challenges. Instrument disinfectants however, must be tested in dirty conditions (Option B) due to the critical nature of the medical device. Disinfectants intended for use in household and commercial properties must be tested according to option C.
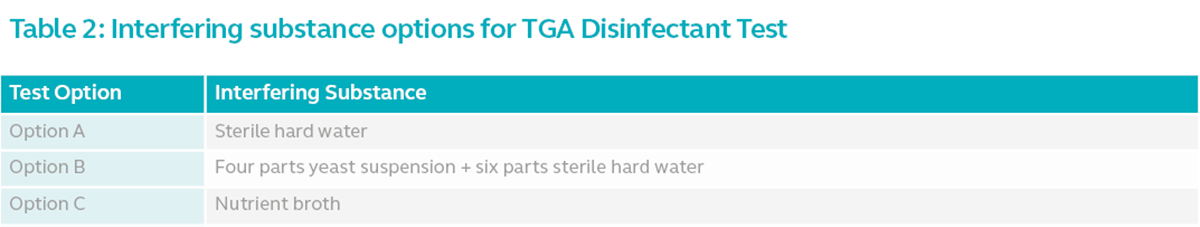
Interfering Substance
The key differentiator between the options is the interfering substance used in the test. Interfering substance refers to the substance used to simulate the possible contaminants that co-occur with microbes in the actual environment.
Challenge
Household and commercial grade disinfectants and skin antiseptics are only required to undergo 1 challenge as indicated in table 1. Hospital grade disinfectants, especially those meant for instruments are subjected to 2 challenges.
In challenge 1, the test disinfectant is diluted according to manufacturer’s recommendation and inoculated with bacterial culture in suspension. Sample is withdrawn from the mixture after 8 minutes of exposure and cultured in five test tubes containing recovery broth and neutralizer. Challenge 1 is passed if no visual growth is observed in at least 2 of the test tubes after 48 hours.
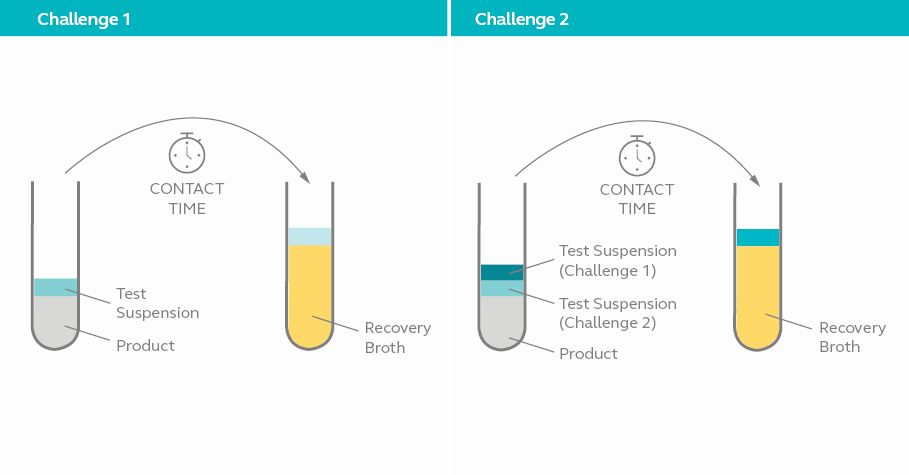
In challenge 2, the test continues after samples are withdrawn in challenge 1 by inoculating additional bacterial culture of the same strain to the remaining mixture. Once again, sample is withdrawn from the mixture after a further 8 minutes and cultured in five test tubes containing recovery broth and neutralizer. Challenge 2 is passed if no visual growth is observed in at least 2 of the test tubes from the second set after 48 hours.

