04 September 2018
Difference in Test Method Between European Norm and TGA Disinfectant Test
The Australian regulation requires disinfectants registered for use in the Land Down Under to pass TGA Disinfectant Test.

At Viroxy, we recently added the TGA Disinfectant Test under our scope of ISO/IEC 17025 accreditation. TGA Disinfectant Test refers to the suspension test method accepted and used in Australia to determine the bactericidal activity of disinfectants. You may be more familiar with its European counterparts;
- EN 13727:2012+A2:2015 – quantitative suspension test for the evaluation of bactericidal activity in the medical area.
- EN 1656:2009 – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants used in the veterinary.
- EN 1276:2009 – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas.
In this post, we attempt to explain the difference between TGA Disinfectant Test and related European Norms.
Background
According to the Australian guideline for disinfectants known as the Therapeutic Goods Order No. 54 (TGO 54), disinfectant manufacturers intending to market their disinfectants in Australia are required to list their products in the Australian Register of Therapeutic Goods (ARTG). Application for registration is subject to evaluation by the Conformity Assessment Branch (CAB) and Therapeutic Goods Laboratories Branch of the TGA, in accordance with Section 25 of Therapeutic Goods Act 1989.
TGO 54 further states that the registered disinfectant must be able to pass TGA Disinfectant Test. Note that it does not state that the disinfectant must have passed the test, just that it should be able to. The Australian regulation in this area is rather vague in that it allows manufacturers to register a disinfectant even if it had only passed a similar suspension test such as EN 13727 and not the TGA Disinfectant Test. It also requires the disinfectant to have passed a carrier test. This is interesting because TGO 54 does not include a carrier test method, only suspension test. As a result, the application for ARTG listing must include the results of carrier tests conducted according to other standards such as the European Norms or AOAC.
However, should TGA sample the product for substantiation of label claims at some point after the registration, the authority will perform the TGA Disinfectant Test. And should the product fail the test, the manufacturer is liable to legal actions regardless of the carrier test results submitted during application.
It then makes sense for manufacturers intending to market their disinfectants in Australia to test their products according to (and pass) TGA Disinfectant Test before listing their products with ARTG.
The question for most European disinfectant manufacturers is, if a disinfectant has passed quantitative suspension test for bactericidal activity according to the European Norms in a specifi c application area, what is the likelihood that it might fail the TGA Disinfectant Test?
For the answer, we contacted TGA.
The Australian authority deem TGA Disinfectant Test as more stringent on vegetative bacteria than EN tests.
Armed with this input from TGA and their comparative analysis, we probed further for a definitive answer on disinfectant tests. But first, let’s explore how TGA Disinfectant Test is carried out.
Product Class and Test Options
According to TGO classification, a disinfectant might fall into one of 3 classes i.e. hospital grade, household or commercial grade and antiseptic. Depending on the class of the product, the test is carried out with 1 of 4 test options. The options available for each class of product is summarised below.
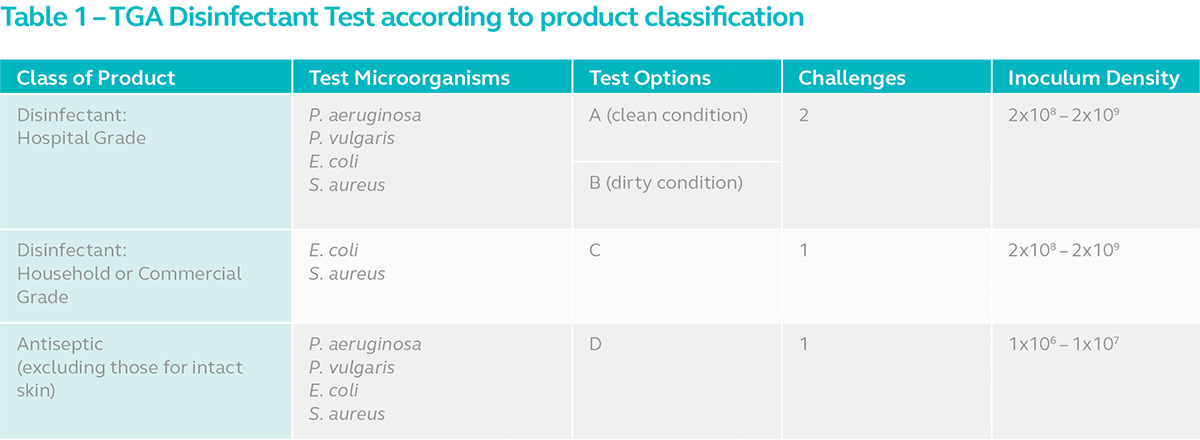
Hospital grade disinfectants or disinfectants intended for use in healthcare facilities are to be tested according to Option A or B. Note however, that instrument disinfectants must be tested according to Option B. Disinfectants intended for use in household or commercial properties must be tested according to Option C and antiseptics, according to Option D.
The main difference between the options is the interfering substance used.
Option A: Sterile hard water
Option B: Four parts yeast suspension + six parts sterile hard water
Option C: Nutrient broth
Option D: Sterile hard water, diluted 1:100 with hard water and 8 ml of final dilution added to 2 ml of sheep serum
TGA Test Method
As indicated in the table, household, and commercial grade disinfectants and skin antiseptics are only required to pass challenge 1. Hospital grade disinfectants especially for instruments, are subjected to more rigorous tests for obvious reasons. They are required to pass two challenges instead of just one.
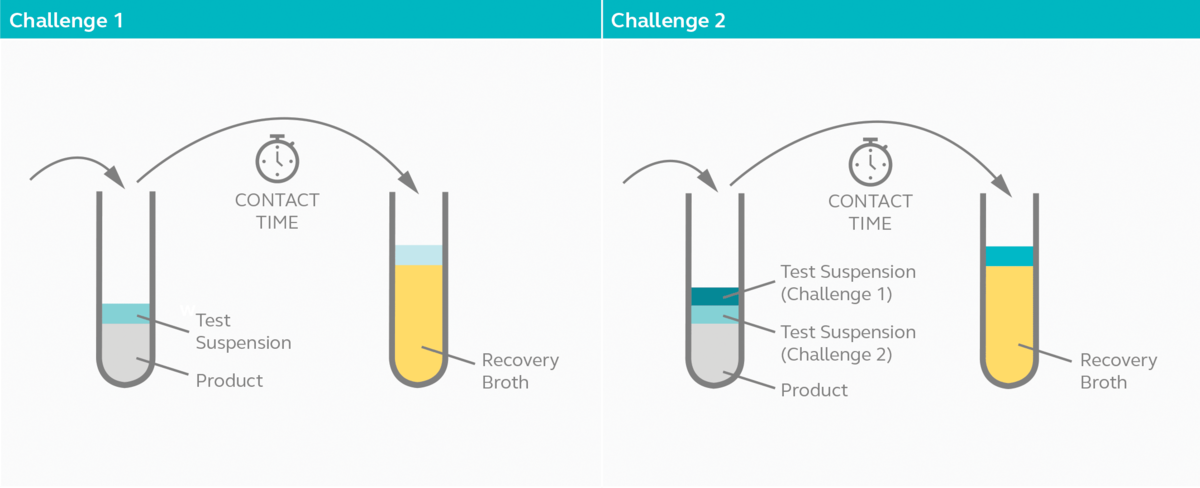
In challenge 1, 3 ml of disinfectant is diluted according to manufacturer’s recommendation on the label and inoculated with 1 ml of bacteria culture in suspension. Five drops are withdrawn from the mixture after 8 minutes of exposure and cultured in five separate test tubes containing recovery broth and appropriate neutralizer. Challenge 1 is passed if no visual growth is observed in at least two of the recovery broths after 48 hours.
In challenge 2, the test continues after samples are withdrawn from the mixture in challenge 1 by inoculating an additional 1 ml of bacterial culture to the remaining mixture. Again, five drops are withdrawn from the mixture after a further 8 minutes and cultured in five test tubes containing recovery broth and appropriate neutralizer. Challenge 2 is passed if no visual growth is observed in at least two of the recovery broths from the second set after 48 hours.
The Difference
So how is it different from the suspension tests according to the European Norms? This is where it gets interesting. Refer to the table for the comparison.
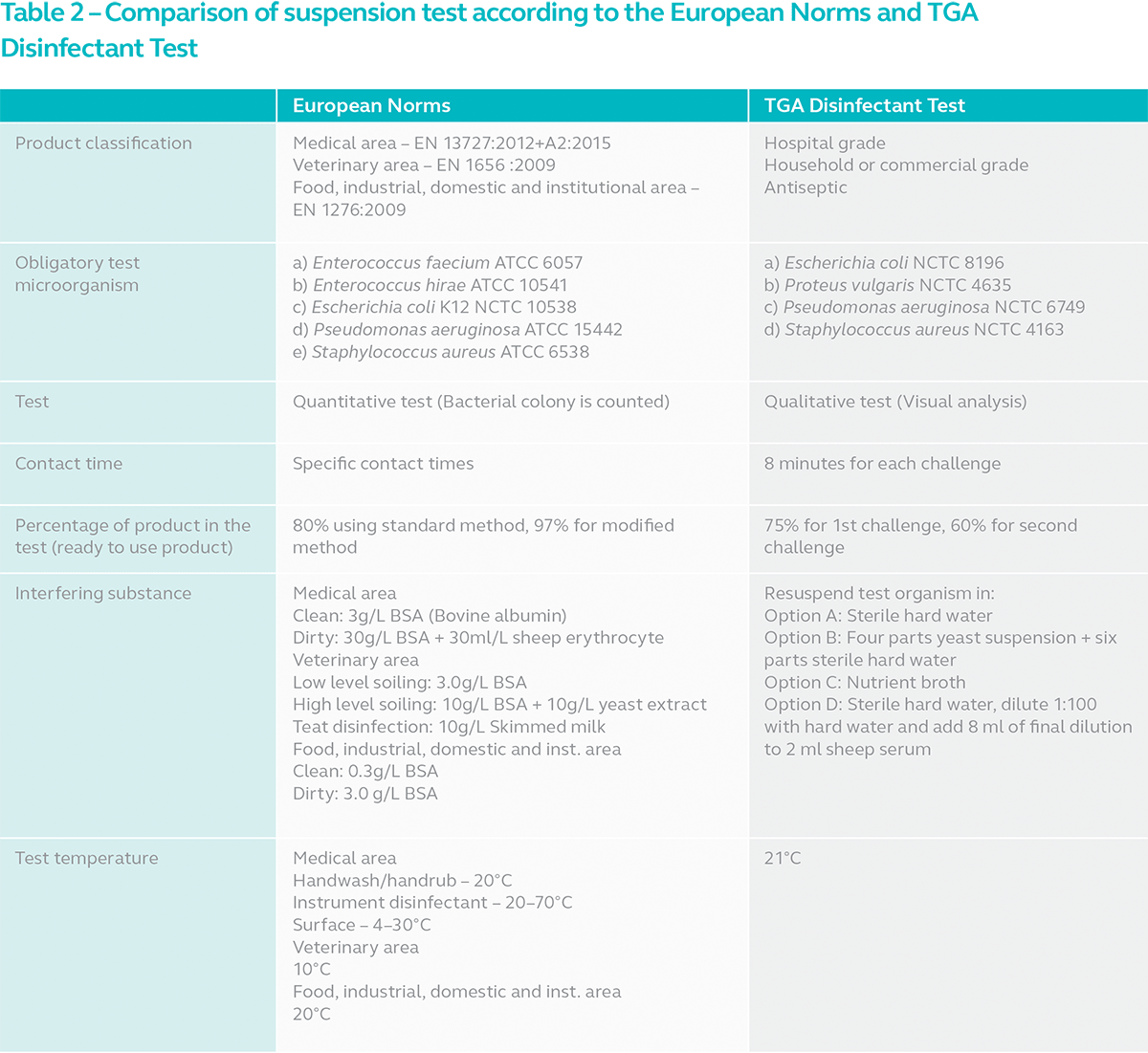
TGA Disinfectant Test is essentially a collection of suspension tests carried out with different interfering substances to determine the bactericidal activity of a disinfectant. Viewed strictly from the scope of suspension tests, it is possible to echo TGA’s sentiment that TGA Disinfectant Test is more stringent than suspension tests carried out according to the European Norms. Mainly due to 2 reasons:
1. Contact time
While tests conducted according to the European Norms use the product’s contact time, TGA Disinfectant Test employs a standard 8-minute across the board. This can be a problem for products with longer exposure times such as 10 or 15 minutes. The product might not effectively kill the test bacteria by the time sample is withdrawn, neutralized and cultured in recovery broth.
2. Dilution
All suspension test methods subject disinfectants to the injustice of further dilution during testing. It is impossible to maintain product concentration at 100% as determined by the manufacturer. Since test microorganisms and interfering substances are also in suspension, further dilution is inevitable. With EN suspension tests, the product concentration is maintained at 80%. With modified methods, it is possible to achieve 97% concentration. With TGA Disinfectant Test however, the product concentration is reduced to 75% in challenge 1 and 60% in challenge 2. This over-dilution greatly reduces the efficacy of disinfectants although it may be perfectly effective at 100% concentration. But there is no way to tell and TGA Disinfectant Test certainly poses a greater challenge compared to EN suspension tests.
Once again, viewed in this very narrow scope of suspension test, it might seem more challenging to pass TGA Disinfectant Test compared to the equivalent European suspension tests. Note however, that TGA Disinfectant Test is conducted at 21°C regardless of the disinfectant grade. Suspension tests according to the European Norms are carried out at different temperatures depending on the industry as well as the application area. Generally, it is lower than the temperature specified by TGA. The significance of this is that lower temperature reduces the effectiveness of the disinfectant. The lack of energy makes it harder for the active ingredients to achieve chemical reaction. Additionally, suspension test is not the only test required for the determination of bactericidal activity according to the European Norms in most cases. We will explore more of that in the next section.
Phase 2, Step 2 Test
In the EU, suspension tests are considered as Phase 2, Step 1 tests. Additional tests such as carrier and surface tests are required for registration of products in the EU depending on the area of use. These additional tests, known as Phase 2, Step 2 tests, simulate actual conditions of use in each application area and serve as a true yardstick for bactericidal activity. As stated at the beginning of this post, although TGO 54 does not include its own version of carrier test, the Australian authority requires and accepts carrier tests conducted according to the European standards or the AOAC. These carrier and surface tests are inherently more difficult to pass than the TGA Disinfectant Test.
Let’s deep dive and analyse these European tests under each classification.
1. Medical Area
Within the medical area, disinfectants are sub-divided into hygienic handwash/rub, surgical handwash/rub, surface disinfection, instrument disinfection and textile disinfection. The disinfectant for each application area must pass EN 13727 (Phase 2, Step 1) suspension test. Additionally, the disinfectants must pass Phase 2, Step 2 tests as indicated in the table below except for textile disinfectants:
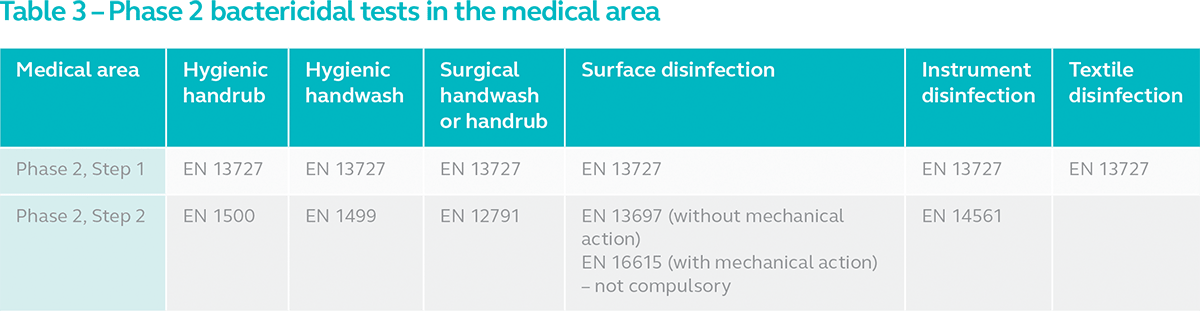
Hand disinfectants are tested on actual human hands in Phase 2, Step 2 tests using standard handwash/rub method widely used by healthcare professionals. For surface disinfectants intended for the medical area, Phase 2, Step 2 tests can be performed with or without mechanical action. EN 13697 is a carrier test performed on surface disinfectant solutions that do not require mechanical action. EN 16615 on the other hand, tests pre-soaked disinfectant wipes with weight application to simulate wiping motion. EN 16615 is not compulsory at the moment, but it is expected to be soon. To know more about this test, read our previous post EN 16615:2015 - One Step Closer to Evaluating the Efficacy of Disinfectant Wipes Better. Instrument disinfectants too are subjected to carrier test EN 14561. We covered the difference between suspension and carrier tests in our article Suspension Test and Carrier Test for Disinfectants According to the European Norms.
2. Veterinary Area
In veterinary, disinfectants are sub-divided into general surface disinfection, teat disinfection, disinfection of equipment by immersion and hygienic handwash/rub and surgical handwash/rub. General surface and instrument disinfectants are subjected to Phase 2, Step 2 tests as indicated in the table below. Handwash/rub products are subjected to tests similar to the medical area.
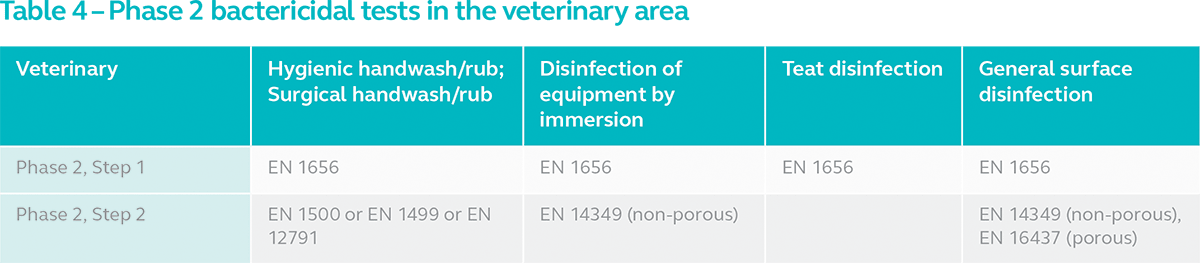
Equipment and general surface disinfectants are subjected to EN 14349, a surface test carried out on non-porous material. For general surface disinfectants in veterinary, there is also EN 16437, an alternative test for porous surfaces. Only teat disinfectants are not subjected to a Phase 2, Step 2 test.
3. Food, Industrial, Domestic and Institutional Area
Disinfectants for the food, industrial, domestic and institutional area are sub-divided into products used for ‘cleaning in place’, hygienic handwash/rub, wipes, products for use in breweries, products for use in the beverage and soft drinks industry and products for use in dairies. The Phase 2, Step 2 test for products intended for use in breweries, beverage and soft drinks industry and dairies is EN 13697, a non-porous surface test.
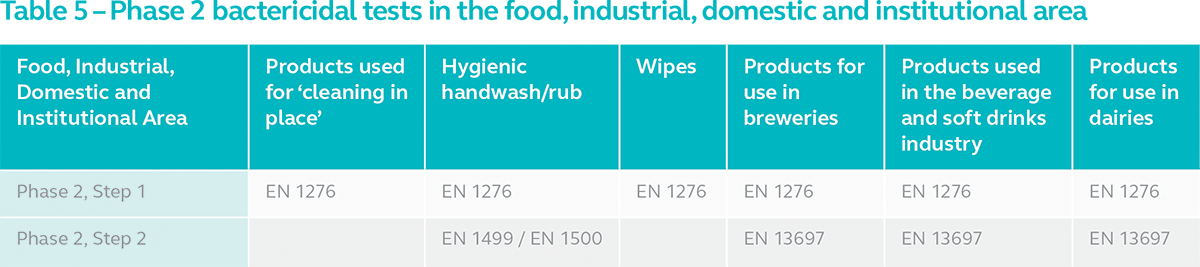
Based on the analysis above, we can conclude that suspension test alone is not sufficient in determining the bactericidal activity of every type of disinfectant. Some require carrier or surface tests which are more representative of actual conditions in specific areas of use. This depends on the area of use and purpose. It is often revealed that the level of concentration required to pass a suspension test is not sufficient to pass a carrier test due to the test method replicating actual conditions. Our advice is for manufacturers to conduct suspension and carrier tests according to the European Norms and supplement them with TGA Disinfectant Test if marketing the product in Australia.
Download PDF file
