16 October 2018
Hand, Foot and Mouth Disease
Viroxy takes a look at how disinfectant manufacturers can help parents prevent hand, foot and mouth disease from spreading.

In 2012, a ‘mystery disease’ appeared in Cambodia killing most patients shortly after admission to hospital. The disease was later identified as hand, foot and mouth disease (HFMD), caused by enterovirus 71, a non-enveloped virus. Today, the disease is still prevalent among children but easily recognisable. Although outbreaks of HFMD occur every few years around the world, in tropical and subtropical countries, they occur year-round. Since the late 1990s, the number of cases has increased in Asia which is a cause for concern.
Current situation
According to the World Health Organization (WHO), a total of 377 629 HFMD cases were reported between 1 and 31 July 2018 in China, resulting in 4 deaths. In Malaysia where Viroxy is based, the Malaysian health officials reported 27,296 cases as of June 2018 compared to just 21,303 in the same period in 2017. Elsewhere, sporadic outbreaks have been reported from time to time.
Pathogens
HFMD is caused by viruses in the enterovirus group which include poliovirus, coxsackievirus and echovirus. Two specific viruses however, are responsible for HFMD and they are enterovirus 71 (EV71) and coxsackievirus A16 (CA16). Infections caused by CA16 are typically self-limiting, but infections caused by EV71 might lead to serious complications resulting in death. Transmission occurs through cough, sneeze or direct contact with an infected person’s blister fluid or faeces.
Symptoms
HFMD commonly affects infants and children in schools, child cares centre and nurseries. Fever is usually the first symptom, lasting 24-48 hours followed by sores in the mouth, rash or blisters on hands, feet and buttocks. In some, it may cause diarrhoea and neurological diseases. Adults can pass the virus to others without showing any symptoms.
Medication
There is no treatment for HFMD and most patients recover fully after an acute illness within 7 to 10 days without medical intervention. However, to relieve pain and fever, patients are prescribed acetaminophen or ibuprofen. HFMD caused by EV71 however, may potentially lead to complications including neurological, cardiovascular and respiratory problems. Some cases prove to be fatal.
Prevention
The risk of infection can be reduced by following Centers for Disease Control and Prevention’s (CDC) guidelines:
- Wash hands often with soap and water especially before eating.
- If soap and water are not available, clean hands with a hand sanitiser containing at least 60% alcohol.
- Disinfect dirty surfaces and soiled items.
- Avoid touching the eyes, nose or mouth. If necessary, touch them only with clean hands.
- Cover mouth and nose with a tissue or sleeve (not hands) when coughing or sneezing.
- Avoid close contact such as kissing, hugging or sharing eating utensils or cups with infected people.
Now we know all the pertinent details of HFMD, let's explore how this domestic problem is connected to you as a disinfectant manufacturer. True, the issue may be of more concern to parents with young children and infants, but it is more relevant to you than you think. And no, we are not appealing to you to develop a special anti-HFMD product. The clue lies in points 2 and 3 in CDC’s guidelines.
Parents with little knowledge in infection control may assume every hand sanitiser and spray disinfectant available in pharmacies is effective against enterovirus 71 and coxsackievirus A16. But as infection control experts, we know there are several salient points to observe where disinfectants are concerned:
- Enterovirus 71 and coxsackievirus A16 are non-enveloped viruses and are therefore more resistant to chemical disinfectants. While CDC recommends hand sanitisers with 60% alcohol content for hand disinfection as a general guideline, it is not recommended to counter specific outbreaks involving non-enveloped viruses such as HFMD. A study published by Günter Kampf in 2017 titled Efficacy of Ethanol Against Viruses in Hand Disinfection in the Journal of Hospital Infection proves that hand sanitisers with 60% alcohol is not effective in inactivating enteroviruses.
- Even if a common hand sanitiser or surface disinfectant is active against non-enveloped viruses, parents need to be advised of the exposure time. This information is not included on the labels of common disinfectants. Without the exposure time, an effective product could still be rendered ineffective due to incorrect use.
We believe manufacturers of hospital grade disinfectants can and should market their product to the public with clear indication of the disinfectant's virucidal activity and the required exposure time to help reduce the transmission risks of HFMD. But are all hospital grade disinfectants capable of inactivating enterovirus 71 and coxsackievirus A16? And is there a sure-fire way to tell?
The short answers to the questions are no and yes respectively. The long answer requires us to turn to the all-important EN 14885:2018. According to this European standard, the virucidal activity of hands and surface disinfectants can be tested according to EN 14476:2013+A2:2019. EN 14476 lists the following obligatory test viruses in determining the virucidal activity of a disinfectant used in the medical area:
- Adenovirus (Non-enveloped virus)
- Norovirus (Non-enveloped virus)
- Poliovirus (Non-enveloped virus)
- Vaccinia virus (Enveloped virus)
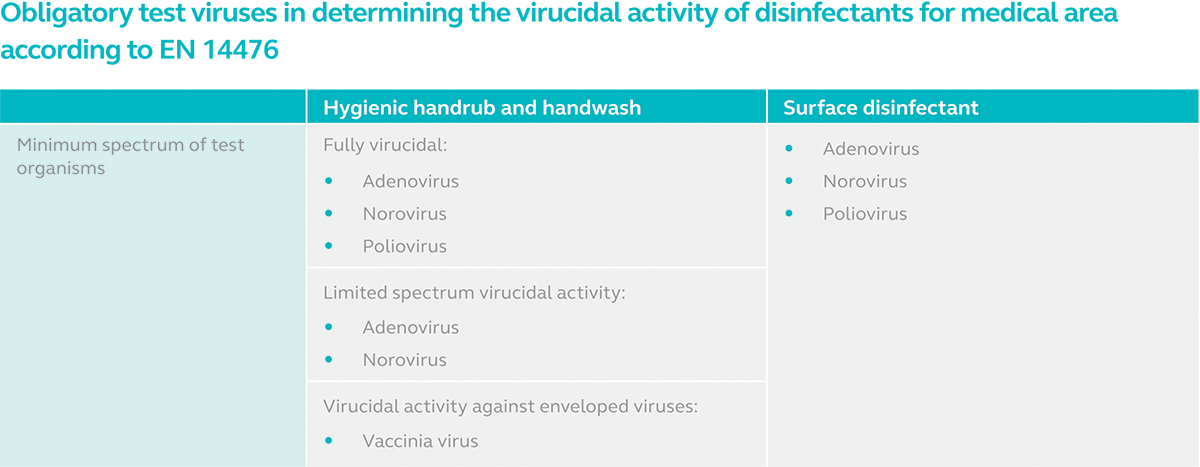
If EN 14476 is performed against all three non-enveloped viruses above for a hand product and it passes the test for adenovirus and norovirus but fails against poliovirus, the product is deemed as limited spectrum virucidal. For a hand product to be fully virucidal or acknowledged as capable of inactivating all viruses including enveloped and non-enveloped viruses, it must be effective against adenovirus, norovirus and poliovirus. Surface disinfectants however, must pass the test against all three non-enveloped viruses to be categorised as fully virucidal.
Note however that EV71 and CA16 are not obligatory strains listed in EN 14476. So, does a positive EN 14476 test result against adenovirus, norovirus and poliovirus mean that the manufacturer can claim the product to be effective against HFMD? The answer is a confusing yes and no but there is an explanation for both.
Yes, the claim is possible: Poliovirus, which is the most resistant among non-enveloped viruses, also belongs to the Enterovirus genus of the Picornaviridae family. This is the same genus as EV71 and CA16. Therefore, technically, a disinfectant tested effective against poliovirus is capable of inactivating both EV71 and CA16. But is this always the case in reality? We can’t tell for sure. Other test methods such as RKI guidelines stretch beyond EN 14476 and include Polyomavirus SV40, another non-enveloped virus to increase the likelihood of the product in inactivating all viruses.
No, the claim would not pass muster: The fact remains that the claim is merely a blanket statement and the product is not proven effective against the two specific microorganisms. We always recommend our clients to test the virucidal capability of a disinfectant with the actual strain(s) when striving for specific claims such as anti-HFMD. An actual test will provide manufacturers the scientific evidence needed to support and back your claims.
We hope the above clarifies some of the vague areas surrounding the issue and provide you the impetus to test your existing products for virucidal activity to prevent the spread of HFMD. EV71 is one of the strains among 164 microorganisms now available at Viroxy for all your testing needs.
Download PDF file
